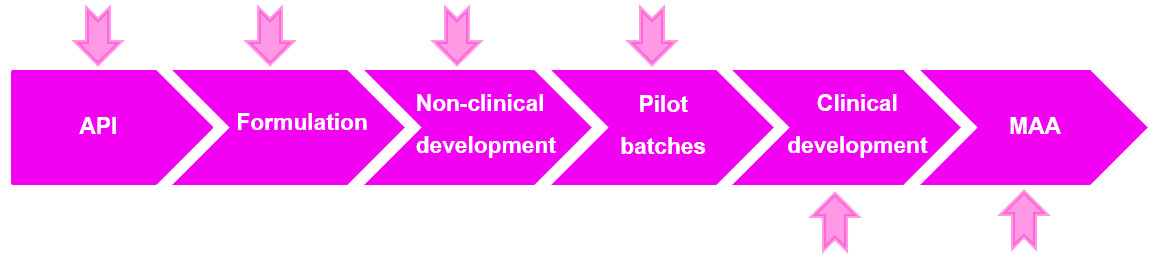
Octalia Technologies supports your R&D teams in...
- Project management (timelines, CMO/CRO follow -up, documentation review/writing...)
- Pre-formulation/Formulation consulting
- Investigation study design (feasibility, stress, packaging screening...)
- Analysis (documention review/writing for method validation, analytical transfer...)
- Stability (study design, data assessment, documentation writing...)
- GLP supplies consulting (manufacturing, specifications, stability, phase study documentation/report for test item formulation and characterization, standard operating procedures...)
- Documentation review/writing (manufacturing, technical, scale-up...)
-
Pharmaceutilcal file writing :
- IND/IMPD
- CTD
- Update of CMC sections
- RFI answers

